Materials
Year 5 & 6
Materials for kids Year 5 & 6 explained. Learning in Key Stage 2 Science. Learn about dissolving, separation and irreversible changes.
Pick a level
Comparing materials by their properties
Materials can be compared and grouped by looking at their properties. Properties are the features of a material that tell us how it behaves and what it is useful for. Scientists study properties to decide which material is best for a particular job.
Some important properties include:
- hardness
- transparency
- solubility
- conductivity
- magnetism
By comparing these properties, we can explain why materials are used in different ways.
Hardness
Hardness describes how difficult a material is to scratch, bend or break.
- Hard materials are strong and resist scratching.
- Soft materials are easier to scratch, bend or wear away.
For example, steel is very hard, which makes it useful for tools and bridges. Rubber is softer, which makes it useful for tyres and grips.
Transparency
Transparency describes how much light can pass through a material.
- Transparent materials let most light through (like clear glass).
- Translucent materials let some light through but not clear images (like frosted glass).
- Opaque materials do not let light through at all (like wood or metal).
This property helps us decide which materials are suitable for windows, lampshades, or curtains.
Solubility
Solubility is about whether a material can dissolve in a liquid, usually water.
- Soluble materials dissolve to form a solution.
- Insoluble materials do not dissolve.
For example, sugar dissolves in water, but sand does not. This property is important when making drinks, medicines, or separating mixtures in science experiments.
Conductivity
Conductivity describes how well a material allows electricity or heat to pass through it.
-
- Metals like copper and aluminium are good conductors. Conductors let electricity or heat pass through it easily.
- Materials like plastic, rubber and wood are poor conductors and are called insulators. Insulators do not let electricity or heat pass through easily.
This is why electrical wires are made from metal but covered in plastic to keep us safe.
Magnetism
Magnetism describes whether a material is attracted to a magnet.
- Some metals, such as iron and steel, are magnetic.
- Most materials, like plastic, wood and glass, are not magnetic.
This property is useful for sorting materials and is used in everyday technology such as motors, door catches and recycling systems.
Dissolving and making solutions
When a solid dissolves in a liquid, it forms a solution. The solid does not disappear. Instead, it spreads out into tiny particles that mix evenly with the liquid.
Some materials are soluble, which means they can dissolve in water. Others are insoluble, which means they cannot.
For example, when you add sugar to water and stir it well, the sugar dissolves. You can no longer see it, but it is still there. The water and sugar together form a sugar solution.
 BBC Bitesize - What is irreversible changes?
BBC Bitesize - What is irreversible changes?Watch the video to learn about irreversible changes then test yourself with the interactive sorting game.
Mixtures
A mixture is made when two or more materials are mixed together but do not dissolve.
You can mix solids with other solids. For example, if you mix sand and pebbles, you have made a mixture. Each material stays the same and can usually be separated again.
You can also have mixtures of solids and liquids, such as sand and water.
Separating mixtures
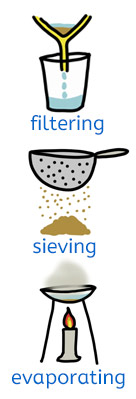 Scientists often need to separate mixtures to study the materials more closely. Different methods are used depending on the materials involved.
Scientists often need to separate mixtures to study the materials more closely. Different methods are used depending on the materials involved.
Filtering
Filtering separates an insoluble solid from a liquid. The liquid passes through the filter, but the solid is left behind. This method is used to separate sand from water.
Sieving
Sieving separates larger solids from smaller solids using a sieve or mesh. For example, sieving can separate pebbles from sand.
Evaporating
Evaporating separates a dissolved solid from a liquid. When the liquid is heated or left in a warm place, it turns into a gas and escapes. The solid is left behind. This method can be used to get salt from salty water.
Try it!
Mix some salt into warm water until it dissolves. Leave the solution in a safe, warm place and watch what happens over time. What do you notice when the water evaporates?
 BBC Bitesize - What is separation?
BBC Bitesize - What is separation?Watch the video to learn about separation then test yourself with the interactive sorting game and quiz.
Reversible and irreversible changes
When materials change, the change can be reversible or irreversible.
Reversible Changes
 A reversible change is one where the material can be changed back again. The material stays the same, even though it may look different.
A reversible change is one where the material can be changed back again. The material stays the same, even though it may look different.
Examples of reversible changes include:
- Melting a solid into a liquid
- Freezing a liquid into a solid
- Dissolving sugar in water (the sugar can be recovered by evaporation)
In reversible changes, no new material is made.
Irreversible Changes
 An irreversible change is one where the material cannot be changed back to what it was before.
An irreversible change is one where the material cannot be changed back to what it was before.
Examples of irreversible changes include:
- Burning paper or wood
- Baking a cake
- A reaction between bicarbonate of soda and acid
These changes usually create new materials with different properties.
 BBC Bitesize - What is irreversible changes?
BBC Bitesize - What is irreversible changes?Watch the video to learn about irreversible changes then test yourself with the interactive sorting game.
New materials and chemical changes
When a change produces new materials, it is called a chemical change. Chemical changes are usually irreversible.
For example, when something burns, it turns into ash, smoke and gases. These new materials cannot be turned back into the original object.
Chemical changes often involve:
- a colour change
- heat or light being released
- gas being produced
Choosing materials for specific uses
 Scientists and engineers choose materials by testing their properties and using evidence from fair tests. This helps them decide which material is best for a particular job.
Scientists and engineers choose materials by testing their properties and using evidence from fair tests. This helps them decide which material is best for a particular job.
For example:
- Copper is used for electrical wires because it conducts electricity very well.
- Glass is used for windows because it is transparent and lets light through.
- Plastic is used for handles and plugs because it is an insulator and helps keep us safe.
Choosing the right material helps make objects stronger, safer and more useful.